STABILITY STORAGE

Custom Condition Stability Chambers
Kryosphere offers a wide variety of cGXP compliant services to ensure that the materials you entrust to us will remain safe and secure. Our Custom Condition Stability Chambers Offer Your Product A Wide Range Of Conditions.
Contact Us
Storage and Stability Conditions
ICH guidelines establish a standardized stability test conditions for global pharmaceutical stability. We can provide expert management across different climatic zones and product categories through strict adherence to these guidelines.
Long-term testing conditions
ICH guidelines are delineated by temperature and humidity levels to ensure a product’s global stability throughout its shelf life. These studies typically require storage at 25°C/60% RH for 12 months, or 30°C/65% RH for the same period. Kryosphere’s advanced biorepository supports these long-term stability studies, providing controlled environments for pharmaceutical and biological products.
Our state-of-the-art stability chambers accommodate any intermediate ICH testing requirements, ensuring that your product and samples remain compliant.

We offer accelerated testing environments to help pharmaceutical companies meet ICH guidelines and expedite product validation for global markets.
Certain products, such as biologics or APIs, require specialized storage conditions. Our custom-designed storage solutions include ultra-low temperatures (-80°C) for biological samples like DNA and plasma, -20°C for biologics and reference standards, and 2-8°C refrigeration for APIs and injectable trial batches. We also offer stability storage at 25°C/60% RH and 30°C/65% RH for medical devices and trial products.

Our expertise in managing stability studies ensures that products, whether pharmaceutical, biological, or medical devices, are stored under precise conditions, compliant with ICH standards and FDA regulations.
Contact Us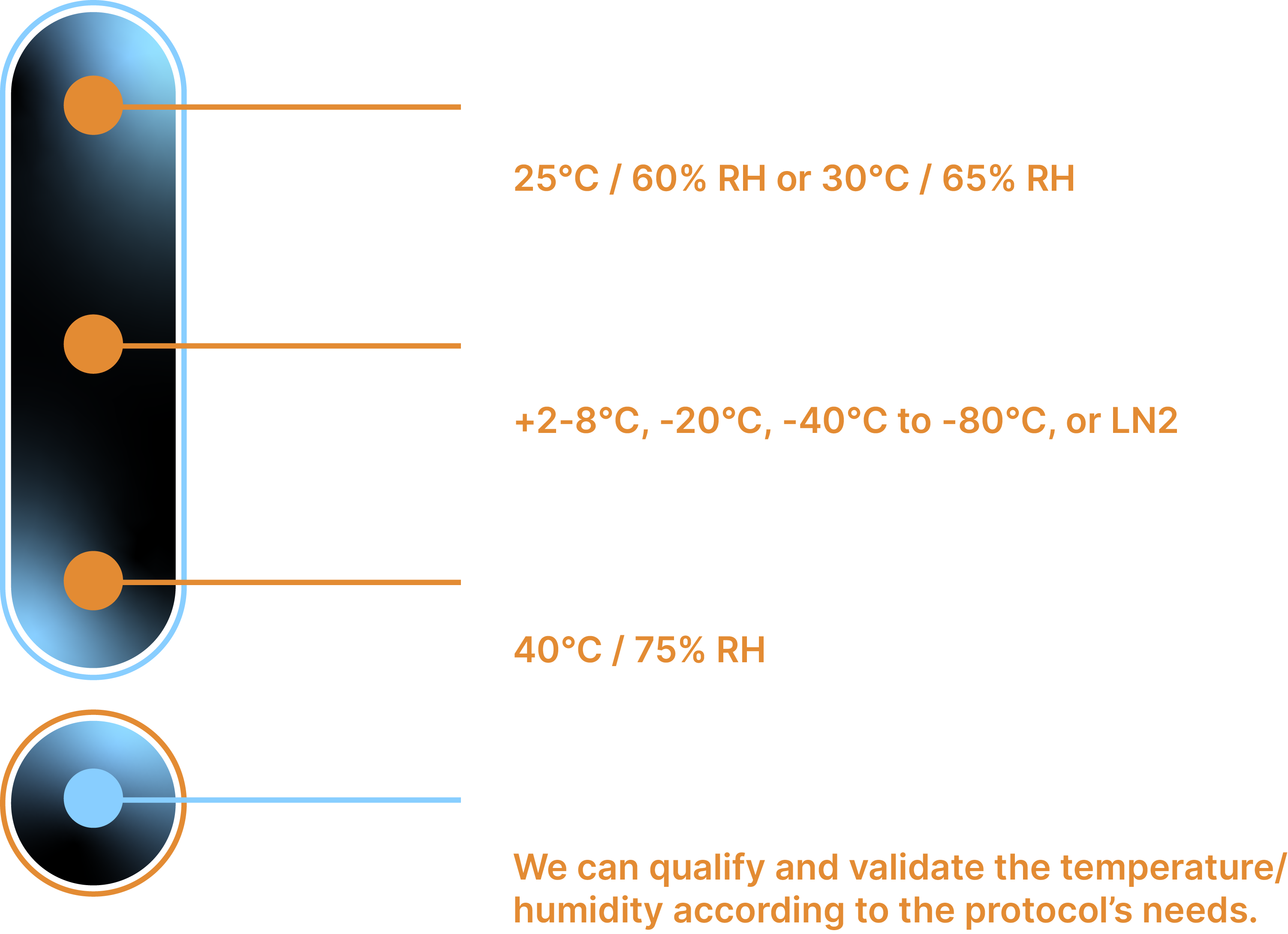
The Quality Commitment
As a leading full-service biomaterial management organization (BMO), Kryosphere consistently delivers high-quality biorepository and stability storage services. To ensure this level of quality, we’ve implemented several key processes:
What is stability storage and why is it important for pharmaceuticals and biologics?
Stability storage is an important component of pharmaceuticals and biologics. It ensures that products remain safe, effective, and of high quality throughout their intended shelf life.
How do you ensure compliance with regulatory requirements for stability storage?
Kryosphere’s biorepository is specifically designed for storage of biomaterials under FDA’s GLP/GMP regulatory compliance. The facility is designed with multiple layers of redundancy and monitoring for all critical systems.
What types of stability conditions can you accommodate (e.g., temperature, humidity, light)?
Our stability storage facilities monitor temperature and humidity within standard ICH ranges, including 25°C/40%RH, 25°C/60%RH, 30°C/65%RH, and 40°C/75%RH.
Can you accommodate ICH stability storage requirements?
Kryosphere specializes in ICH compliant stability storage and can support clients in developing stability protocols while providing guidance on the timely transfer or shipment of materials for critical timepoint tests. Our stability storage facilities monitor temperature and humidity within standard ICH ranges, including 25°C/40%RH, 25°C/60%RH, 30°C/65%RH, and 40°C/75%RH.
What are your capabilities for managing stability storage of biologics and sensitive pharmaceuticals?
Kryosphere provides controlled temperature storage at all temperatures – from controlled ambient to Liquid Nitrogen Liquid and Vapor Phase.
Stability Storage Guidelines
We offer a wide range of storage solutions that satisfy the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) storage condition guidelines.
What are ICH Stability Guidelines?
The ICH (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use) stability guidelines are critical for determining and maintaining efficacy in pharmaceutical products. These guidelines ensure that pharmaceuticals retain their potency, safety, and effectiveness under various environmental conditions.
We adhere strictly to the ICH guidelines, ensuring that your products are maintained all the way from research to market release. We ensure that your products meet global regulatory expectations for quality, efficacy, and patient safety.
For pharmaceutical companies, partnering with Kryosphere to adhere to ICH stability guidelines is not just a regulatory requirement—it’s a vital part of ensuring that products are delivered to patients with uncompromised quality and reliability.
Interested in KRYOSPHERE?
Do you need to challenge the efficacy and reliability of your finished or intermediate drug product? Inquire with us today!

