Biostorage Services

Confidence At All Temperatures
Kryosphere offers a wide variety of cGXP compliant services to ensure that the materials you entrust to us will remain safe and secure.
We have over a decade of experience in storing your irreplaceable material. Our goal is to provide unmatched customer service.
Contact UsKryosphere offers general and dedicated storage for all industry standard temperature set-points. We offer storage for ambient, controlled room temperature ambient, +4°C, -20°C, -40°C, -70°C, -80°C, vapor phase liquid nitrogen, and ICH compliant stability storage. With custom warm and cold alarm thresholds based on client and material needs, you can have confidence knowing your material is being stored and handled by the best in the industry.
All refrigeration equipment is backed up by our own onsite licensed refrigeration staff.
Contact Us
Security at all temperatures
We offer storage for ambient, controlled room temperature ambient, +4°C, -20°C, -40°C, -70°C, -80°C, vapor phase liquid nitrogen, and ICH compliant stability storage.
CONTACT US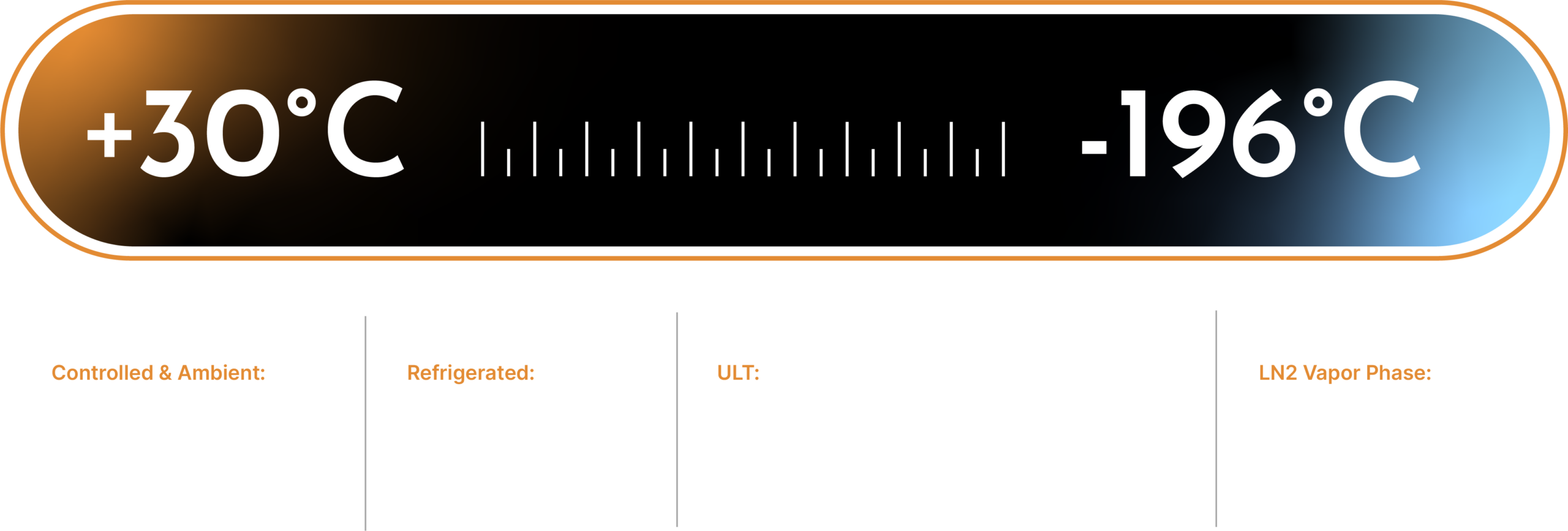
+15°C to +30°C
Controlled & Ambients:
Retains, Buffers, Reagents, WFI Water, Manufacturing Disposable, Resins, Medical Devices, Lab Equipment
-80°C to -60°C, -20°C to -40°C
ULT:
Bulk Drug Substances, Samples, Retains, Biologic Material, Clinical Trial Samples, Tissue Sample, API
-196°C to -135°C
LN2 Vapor Phase
Clinical trial samples, Biologic Samples, Master Cell Banks, Working Cell Banks, Patient Samples, Tissue Retains
+2˚C to +8˚C
Refrigerated:
Media, Drug Products, Drug Substance, Resins
Biostorage FAQs
Which industries does Kryosphere work with?
Academia, Biopharmaceuticals, Bioagriculture, Biobanking & Clinical Kitting, Clinical Research, Medical Services, Cultured Animal Cells.
What temperature ranges does Kryosphere offer for biostorage?
Kryosphere has the capability to store material at temperatures from controlled ambient, +5°C, -20°C, -40°C, -70°C, -80°C, as well as, liquid nitrogen vapor or liquid phase in controlled temperature units and walk-in cold storage. We provide secure storage areas within the secure facility for sensitive material if needed.
How does your pricing structure work for biostorage services?
Prices range based on temperature/condition products need to be stored at and the footprint of the material.
What are your disaster recovery protocols?
Kryosphere’s business continuity plan (BCP) addresses the procedures that are to be followed in the case of a natural disaster or other catastrophic event. These include, but are not limited to, arrangements with the local fire and police departments and our electrical power company. The BCP addresses issues that may arise either naturally (i.e., a tornado, or flooding), or due to human action (i.e., chemical spill or fire). In addition, Kryosphere is served by one of the area’s most stable power grids and is located outside of the FEMA 500-year flood plain. The Kryosphere facility includes state-of-the-art equipment, experienced and trained personnel, continuous electronic monitoring, 24/7 security, and fully redundant systems.
What are the benefits of offsite storage?
Local clients have the ability to physically tour and audit Kryosphere’s biorepository at any time. Kryosphere allows client material drop-off and pick-up. Client materials are handled through our own in-house cold chain of custody logistics team. The same biotechnicians managing your stored material are also transporting material between client sites and Kryosphere. Thus, assuring you that GMP/GLP regulations are maintained throughout a material transfer.
How do our biorepository services maintain best practices?
An emergency response service providing fast and efficient 24/7 business continuity and disaster recovery. Emergency response couples our specially trained personnel with our mobile biorepository (KryoTRUCK™) and appropriate equipment (i.e., cryo-containers containing dry ice, at-temperature freezers and/or LN2 units, temperature monitors, and KryoCART™) for biomaterial stability assurance.
What types of redundancies and backups do we have in place to safeguard your product?
Backup Systems: All electronic records are maintained on and off-site through an IT hosting service. Original paper records are scanned and electronically archived with all other critical information. All client sample records are retained in the BSI inventory database which is remotely hosted at a highly secure data facility with at least one off-site mirror site. Corporate data and security data are backed up off-site daily by an independent IT hosting service.
How do you ensure the security of stored biomaterials?
The building is protected by alarm, card access and is under 24/7 video surveillance utilizing CCTV. Site-based 24 hr. security cameras, intruder alarms and perimeter fencing. Within the Kryosphere facility, access to the repository portion is restricted to those personnel assigned to biorepository operations. Card-access controls entry and all access records are kept indefinitely. Within the biorepository additional access-control is available at client’s request (caged areas, individual freezer access, secure storage rooms, etc.).
Are your storage facilities cGXP compliant?
Adhering to strict cGxP performance standards, our technicians follow standardized procedures and rigorous quality control measures, providing complete assurance to clients when transferring custody of biological specimens. With a capacity to store and manage over 10 million samples, Kryosphere serves as a leading center for biorepository services, cold chain-of-custody logistics, and clinical trial sample management for clients worldwide.
Do you offer dedicated storage options?
Kryosphere occupies over 135,000 square foot facility of which 80,000 square feet is dedicated to GLP/GMP storage with an additional 35,000 square feet of CRT storage across the street.
How do you handle temperature monitoring and alarms?
Kryosphere’s Cold-Chain-of-Custody logistics services include a full audit trail of material temperature. Direct transfer of bulk material is accomplished using a KryoTRUCK™. This validated “mobile biorepository” is capable of moving fully loaded freezers supplied with electrical power and redundant temperature monitoring systems. Our KryoTRUCKS™ include at least one empty backup freezer on board along with extra dry ice.
How do you manage sample retrieval and chain of custody?
Kryosphere’s Cold-Chain-of-Custody logistics services include a full audit trail of material temperature. Direct transfer of bulk material is accomplished using a KryoTRUCK™. This validated “mobile biorepository” is capable of moving fully loaded freezers supplied with electrical power and redundant temperature monitoring systems. Our KryoTRUCKS™ include at least one empty backup freezer on board along with extra dry ice
Do you offer services for clinical trial sample management?
As a leading full-service biomaterial management organization (BMO), Kryosphere consistently delivers high quality biorepository services (active and archive sample management), cold chain-of-custody logistics (moving individual vials to fully loaded freezers at-temperature), and clinical trial sample management (protocol development, site training, kitting, distribution, and support in accordance with FDA requirements).
How do you handle temperature monitoring and alarms?
Kryosphere’s Cold-Chain-of-Custody logistics services include a full audit trail of material temperature. Direct transfer of bulk material is accomplished using a KryoTRUCK™. This validated “mobile biorepository” is capable of moving fully loaded freezers supplied with electrical power and redundant temperature monitoring systems. Our KryoTRUCKS™ include at least one empty backup freezer on board along with extra dry ice.
Do you offer services for clinical trial sample management?
As a leading full-service biomaterial management organization (BMO), Kryosphere consistently delivers high quality biorepository services (active and archive sample management), cold chain-of-custody logistics (moving individual vials to fully loaded freezers at-temperature), and clinical trial sample management (protocol development, site training, kitting, distribution, and support in accordance with FDA requirements).
How do you ensure compliance with FDA requirements?
Kryosphere provides premier cGXP storage and support services that adhere to FDA regulations and ISBER guidelines, ensuring compliance.
Can you handle both active and archive sample management?
Kryosphere provides high quality biorepository services for both active and archive sample management.
What is your capacity for sample storage?
Kryosphere is specially designed with the capacity to store and manage over 25 million samples and serves as a center for the delivery of biorepository services, cold chain-of-custody logistics, and clinical trial sample management.
Can you track my samples’ history and location in real-time?
A best-of-breed electronic inventory management system from BSI Systems. This sample management access research Tool and laboratory information management system (LIMS) allows the tracking of each individual biomaterial in our custody. All biomaterial received into the repository is entered into LIMS using either unique identifiers already on the specimens or barcoded labels are applied for identification and inventory. BSI Engage web access provides our clients with 24/7 web-based access to their inventory in a validated and secure environment.
How does Kryosphere ensure sample integrity and quality?
As a GLP/GMP facility Kryosphere maintains a rigorous quality assurance program. We have in-house Quality Assurance staff and a complete set of SOPs for all biorepository and logistics processes. Kryosphere’s in-house regulatory closely monitors all biorepository procedures and ensures that all SOP’s are rigorously followed. We welcome regular quality audits from our clients and have never received a negative review.
How do you request sample shipment, transfer, or destruction?
Kryosphere maintains at least one freezer per nominal temperature on standby for receiving shipments. Kryosphere has the ability to obtain additional cold boxes, freezers and LN2 units from our sister company, Scientific Calibration, within a relatively short period of time.
What security measures are in place for the web portal?
Confidentiality is maintained for all of your material. Access to our web-based inventory system is secure and a table of authorities specifies the access granted to each individual. All data is backed up daily by a secure off-site hosting service.
How does Kryosphere support regulatory compliance and audit readiness?
As a GLP/GMP facility Kryosphere maintains a rigorous quality assurance program. We have in-house Quality Assurance staff and a complete set of SOPs for all biorepository and logistics processes. Kryosphere’s in-house regulatory closely monitors all biorepository procedures and ensures that all SOP’s are rigorously followed. We welcome regular quality audits from our clients and have never received a negative review. All activity involving any project material is documented in detail in accordance with Kryosphere SOPs and relevant federal regulations.
What customer support does Kryosphere provide after implementation?
A signed, formal Contract in place, including Service Level Agreement (SLA). Monitoring should occur against the SLA. There should be contracts in place with any other third parties providing support services to the offsite storage company.
Do you provide storage for local & global partners?
Yes, we work with many domestic and international companies.
Interested in KRYOSPHERE?
Our freezers are stringently validated and qualified to store all temperature ranges of material. Inquire about storage with us today!

